Say no to manually filling long application forms
Visit any careers page and a lightning button will pop up on any compatible page with a form
Use ChatGPT to auto-fill job forms
Ask for Referral for any job post
Raksha Raghavan
Quality Assurance Specialist | Quality & Regulatory Affairs at Leica Microsystems
Education Overview
• b. m. s. college of engineering
• sri bhagawan mahaveer jain college
• carmel convent school india
Companies Overview
• leica microsystems
• hcl
• ezemrx
• coeo labs pvt. ltd.
• innaccel
• philips
Experience Overview
6.7 Years
Find anyone’s contact
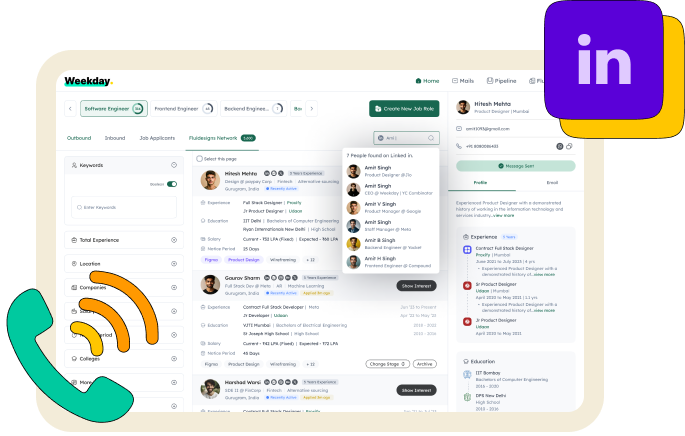
Experience
No data found
Skills
Boost your visibility and stand out to employers with referrals from your LinkedIn connections.
Contact Details
Email (Verified)
xxxxxxxx@xxxx.xxMobile Number
+91XXXXXXXXXXEducation
No data found
Frequently asked questions
Find anyone’s contact and let Weekday reach out to them on your behalf
Start hiring nowStop manually filling job applications. Use AI to auto-apply to jobs
Look for jobs now




